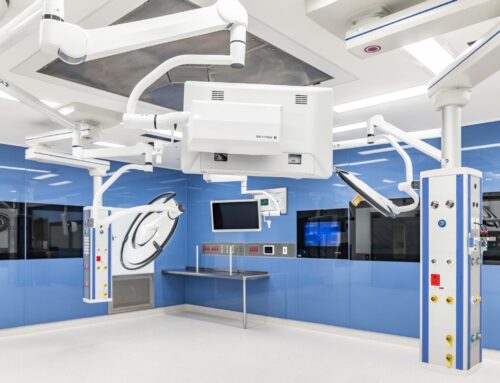
NDY is proud to be the principal design consultant for Project Banksia, an advanced-manufacturing biopharmaceutical facility in Melbourne, Australia. Working closely with CSL Seqirus, our team has delivered a state-of-the-art facility design that will transform vaccine production and address public health demands.
The facility will utilise innovative cell-based technology to produce influenza vaccines for both pandemics and seasonal vaccination programs; the first in the Southern Hemisphere. It will also manufacture crucial products such as antivenom for Australian venomous snakes, spiders and marine creatures and the world’s only human vaccine for Q-Fever. This project holds immense national and global significance, enhancing the global influenza manufacturing supply chain.
NDY’s expertise in building services, structure, specialist disciplines and technical design management led to the successful complete design integration of this complex facility in record time. This includes sustainable design leadership and technical modelling suitable for Australian excellence in design and occupant wellness.
Our team successfully completed the detailed design, passing stage gate approval milestones and meticulously coordinating with procured manufacturing process equipment to target a ‘zero clash’ 3D construction model encompassing all works disciplines. Close collaboration between the client, design team and construction partners has been a highlight throughout the design and construction phases.
The facility will house PC2 and PC3 laboratories, clean and black utilities, goods warehousing, administration offices, staff cafeteria and quality control testing facilities.
As a project delivery partner, NDY is honoured to have played a vital role in this significant project that serves public health on a global scale.
Project Details
Market Sector:
Health & Sciences
Client: CSL Seqirus
Architect: BLP, Wood
Contractor: BESIX Watpac
Completion: 2024