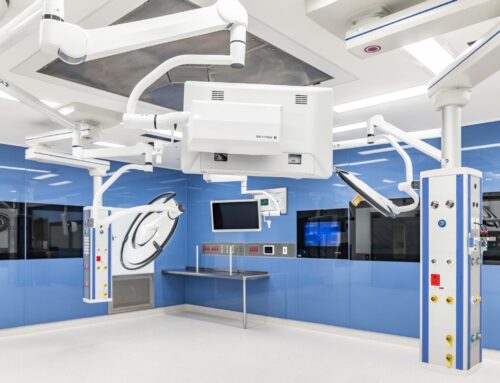
Cyclowest’s new $25 million cyclotron radiopharmaceutical production facility is only the second of its kind in Western Australia. Used to produce therapy radiopharmaceuticals, such as fluorodeoxyglucose (FDG) which is the active ingredient used in PET (positron emission tomography) scans the custom radiopharmaceuticals produced in the new facility will help both public and private sector clinicians to diagnose, evaluate and treat numerous different types of cancers, cardiac conditions, neurological diseases and other medical conditions.
Located in Bayswater, Perth, the privately funded and operated facility consists of a compact 2-storey building with a 600 m2 footprint per floor. It includes a cyclotron, hot cell service area, hot labs, a quality control clean room and other production support accommodation on the ground level, with a technical mezzanine, plant room and office space on the upper level.
The cyclotron unit uses a particle acceleration process to create customised radiopharmaceuticals. The process for producing the radiopharmaceuticals required the equipment to be enclosed within a 900 mm thick concrete wall to create an absorptive barrier to contain radioactive emissions.
The cyclotron itself is self-shielded with large water tanks and protective high density lining in-built within its structure.
As a registered independent supplier and a pharmaceutical infrastructure project, our team worked closely with our client, the architect and contractor in successfully complying with the strict Therapeutic Goods Administration and Good Manufacturing Process (GMP) design, installation and operational qualification criteria (DQ/IQ/OQ). Our mechanical heating, ventilation and cooling, building management systems and lab gases design solutions aligned with strict GMP regulations which aim to help ensure that products are consistently produced and controlled according to quality standards to minimise the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product.
To comply with the regulations the systems included 100% outside-air air conditioning for all process accommodation, HEPA filters supplied air to all GMP classified areas, a strict pressure control regime to contain airborne contaminants in negative-pressure spaces while also maintaining sterility in positive pressure cleanroom classified areas.
An independent system for strict environmental control of pressure, temperature and humidity was also implemented in all areas, with HEPA filters and carbon filtration on all exhaust air systems. The facility also required a suite of laboratory gases for testing and equipment supplies including hydrogen, nitrogen, helium and compressed air.
To deliver on Cyclowest’s sustainability ambitions and our own sustainable design philosophy, NDY’s team designed an all-electric energy plant with heating and cooling provided via new generation 4-pipe heat recovery chiller heat pumps. Significant energy recovery is also achieved via runaround coils recovering energy from all exhaust airstreams. These measures provide an additional sustainability benefit to our client and reduce overall operation costs.
Throughout the project delivery phase, supply chain issues and cost escalation were a main concern. Working to deliver the facility through the pandemic, our team actively managed this to ensure the project was delivered to budget and client requirements.
Our team’s collaboration with our client and stakeholders was imperative to the successful delivery of the new cyclotron facility which will provide life-saving medication and treatment options for local healthcare facilities. The project also provided a unique opportunity for our engineers to work on a rare and complex mechanical design engineering project and emphasized NDY’s focus on providing best-for-project design solutions.
Project Details
Services:
Fire Engineering
Mechanical
Sustainability
Market Sector:
Health & Sciences
Client: Cyclowest
Architect: DKJ
Contractor: Built
Value: $25 m
Completion: 2023